In a fast-paced world, the risks companies are exposed to have to be managed in order to continue to grow and develop, and to evolve quickly. Effectively managed risks help companies achieve their goals.
Products that are developed following quality standards, have improved the quality of life for thousands of people. The idea of improving the quality of life is the premise of product risk management.
If you are developing medical devices in this day and age, you absolutely must have an established Risk Management process that is defined, documented, and implemented.
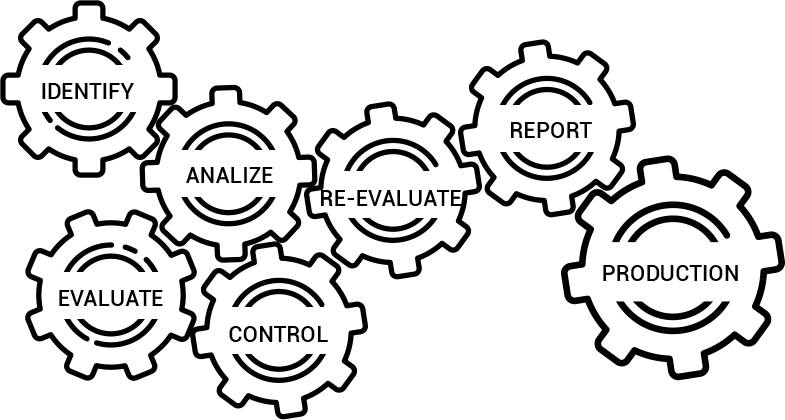
The Risk Management process includes:
- Risk Management Planning – should identify the risk management activities company anticipate and plan throughout the product’s lifecycle.
- Risk Analysis – systematic use of available information to identify hazards
- Risk Evaluation – process of comparing the estimated risk against given risk criteria to determine the acceptability of the risk
- Risk Controls – process in which decisions are made and measures implemented by which risks are reduced to specified levels
- Overall Residual Risk Acceptability – identifying the acceptability of risks that remain after risk control measures have been taken
- Risk Management Reporting – the summarized result of all steps in risk management processes
- Production & Post-Production Information – a system to collect and review information about the medical device in the production and post-production.
A Risk Process describes the steps companies need to take to identify, monitor, and control risk. Within the Risk Process, a risk is defined as any future event that may prevent them to meet their team goals. A Risk Process allows companies to identify each risk, quantify the impact and act to prevent it from occurring and reduce its impact of it. The risk management process is not a single step to be performed during the development of the medical device, but an ongoing activity that is constantly and consistently applied throughout the entire life of the medical device from the beginning to drawing of the medical device from the market.
